Immunoassay for Cyanobacterial Liver Toxins in Surface Water Contaminated by Blue-Green Algae
Background
The contamination of surface water resources by toxin producing cyanobacterial blooms are a global problem. The cyanotoxins, comprising >100 microcystin (MC) and several nodularin (Nod) variants, cause serious short- and long-term health hazards both in humans and animals and have for long been associated with increased frequency of liver cancer in China (Ueno et al. Carcinogenesis 1996).
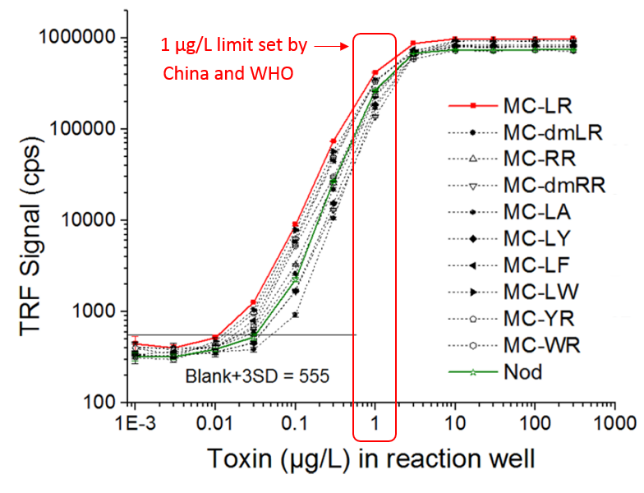
The maximum allowed level of microcystin-LR in drinking water is set at 1 µg/L in China (GB 5749-2006) and also by WHO.
Description
A 2nd generation “sandwich-type” immunoassay is provided that allows broad-spectrum detection of all the relevant cyanotoxins in a single one-step immunocomplex assay.
- Uses the robust non-competitive (two binding site) assay format. Only one site used in 1st gen. assays.
- Significant advances achieved in terms of coverage, accuracy, usability, cost, speed & producibility
- No similar tests on the market thanks to IPR, long term background research and pioneering techniques used
- The test components (antibodies) can be adapted to any immunoassay testing platform (single-use quick tests, automated microtiter-well based tests, etc.)
- Group-specific MC and Nod assays also available upon request (in addition to the broad-spectrum assay)
Application Areas and Potential End Users
Monitoring and on-site testing of drinking or recreational water samples. Potential end users include urban water plants and authorities (high-scale automated testing), aquafarmers, farmers and breeders (for testing of irrigation water or animal drinking water) as well as consumers (single-use quick test formats).
The IP is available for licensing to test developers and testing service providers from the TTO of the University of Turku (UTU)
Title: ANTIBODIES AGAINST IMMUNOCOMPLEXES COMPRISING CYANOBACTERIAL CYCLIC PEPTIDE HEPATOTOXINS
Technology Readiness Level (TRL): 6
Status: The technology and components have been proven in different scales and assay formats. The assay is to be adapted to test platform selected by licensee.
Included in the license: 1) Well-characterized recombinant antibody (PCT patent application PCT/FI2016/050911), recognizing the immunocomplex between the cyanotoxin and the primary antibody (also provided), and 2) the immunocomplex measurement technology by collaborator (CN1711476B).