Diagnostics and treatment of aggressive cancer based on PME-1 expression and siRNA-based silencing
Background
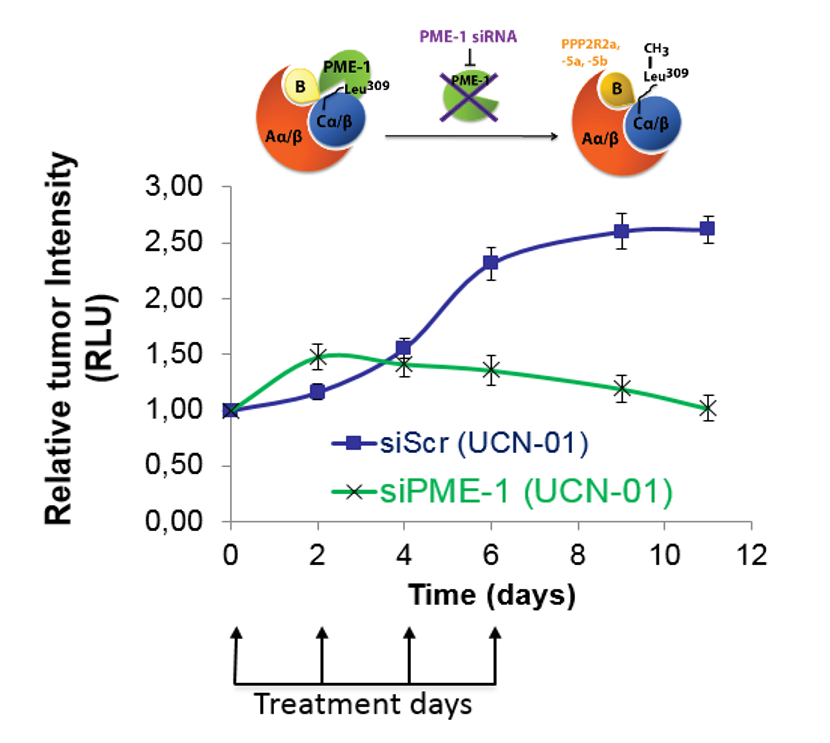
PME-1 (protein phosphatase methylesterase-1) has recently been identified as a potentially significant tumor promoter which acts by inhibiting the major tumor suppressor, protein phosphatase 2A (PP2A). PME-1 inactivates PP2A by de-methylating its catalytic subunit PP2Ac and also by binding it directly.
PME 1 has now been identified as a promising therapeutic target in many aggressive human malignancies, including glioblastoma and endometrial adenocarcinoma.
Description
The current group of inventions provide means for
- diagnosing and stratification of patients based on PME-1 expression
- siRNA-mediated therapy for silencing PME-1
with the following major findings:
- Silencing PME-1 gene sensitizes cancer cells for apoptosis induced by chemotherapeutic agents
- PME-1 expression level can be used to differentiate patients who are likely to benefit from chemotherapy
- There is significant potential for treating human glioblastoma multiforme (GBM), the most common and most aggressive malignant primary brain tumor
- Proven or expected applicability in other cancers with invasive phenotypes
Application Areas
Combination therapy for cancer, sensitization method, personalized medicine, stratification, drug development.
The IP is available for licensing from the TTO of the University of Turku (UTU)
ID: UTU309
Title: METHOD OF SELECTING INDIVIDUALIZED BRAIN CANCER THERAPY
PCT Publication: WO2014033367 (A1)
National Phase: US, EP, JP, CN, CA
Title: COMBINATION THERAPY
PCT Publication: WO2012175798 (A2)
National Phase: US, EP, JP, CN, CA
Status: Technology Readiness Level (TRL) 3-4
Significant evidence for the treatment of glioblastoma in a xenograft mouse model, where PME-1 siRNA combination therapy strongly inhibited tumor growth